Asam nitrit
senyawa kimia
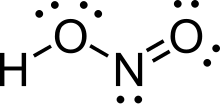
Nitrous acid (molecular formula HNO2) is a weak and monobasic acid known only in solution and in the form of nitrite salts.
| |
| Nama | |
|---|---|
| Nama IUPAC (preferensi)
Nitrous acid | |
| Nama IUPAC (sistematis)
Hydroxidooxidonitrogen | |
| Penanda | |
Model 3D (JSmol)
|
|
| 3DMet | {{{3DMet}}} |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| Nomor EC | |
| Referensi Gmelin | 983 |
| KEGG | |
| MeSH | Nitrous+acid |
PubChem CID
|
|
| Nomor RTECS | {{{value}}} |
CompTox Dashboard (EPA)
|
|
| |
| |
| Sifat | |
| HNO2 | |
| Massa molar | 47,013 g/mol |
| Penampilan | Larutan biru pucat |
| Densitas | Sekitar 1 g/ml |
| Titik lebur | Hanya diketahui dalam larutan |
| Keasaman (pKa) | 3,398 |
| Bahaya | |
| Titik nyala | Tidak mudah terbakar |
| Senyawa terkait | |
Anion lain
|
Asam nitrat |
Kation lainnya
|
Natrium nitrit Kalium nitrit Amonium nitrit |
Senyawa terkait
|
Dinitrogen trioksida |
Kecuali dinyatakan lain, data di atas berlaku pada suhu dan tekanan standar (25 °C [77 °F], 100 kPa). | |
| Referensi | |
Nitrous acid is used to make diazides from amines; this occurs by nucleophilic attack of the amine onto the nitrite, reprotonation by the surrounding solvent, and double-elimination of water. The diazide can then be liberated to give a carbene or carbenoid.
Referensi